Recent Publications
Interview with Dr. de Guglielmo and Dr. Doyle
In this eNeuro “Beyond the Paper” interview, Drs. Giordano de Guglielmo and Michelle Doyle discuss the creation of a large-scale Preclinical Alcohol Biobank built from more than 900 genetically diverse heterogeneous stock rats behaviorally characterized for alcohol use disorder–like traits. Using chronic intermittent ethanol exposure and self-administration paradigms, our team collected tissues such as brain, liver, blood, and others, linked to detailed behavioral and genetic data, creating an open, FAIR-compliant resource freely available to nonprofit researchers. The biobank is designed to accelerate multidisciplinary collaboration, enable multi-omics discovery, and support the development of personalized treatments for addiction
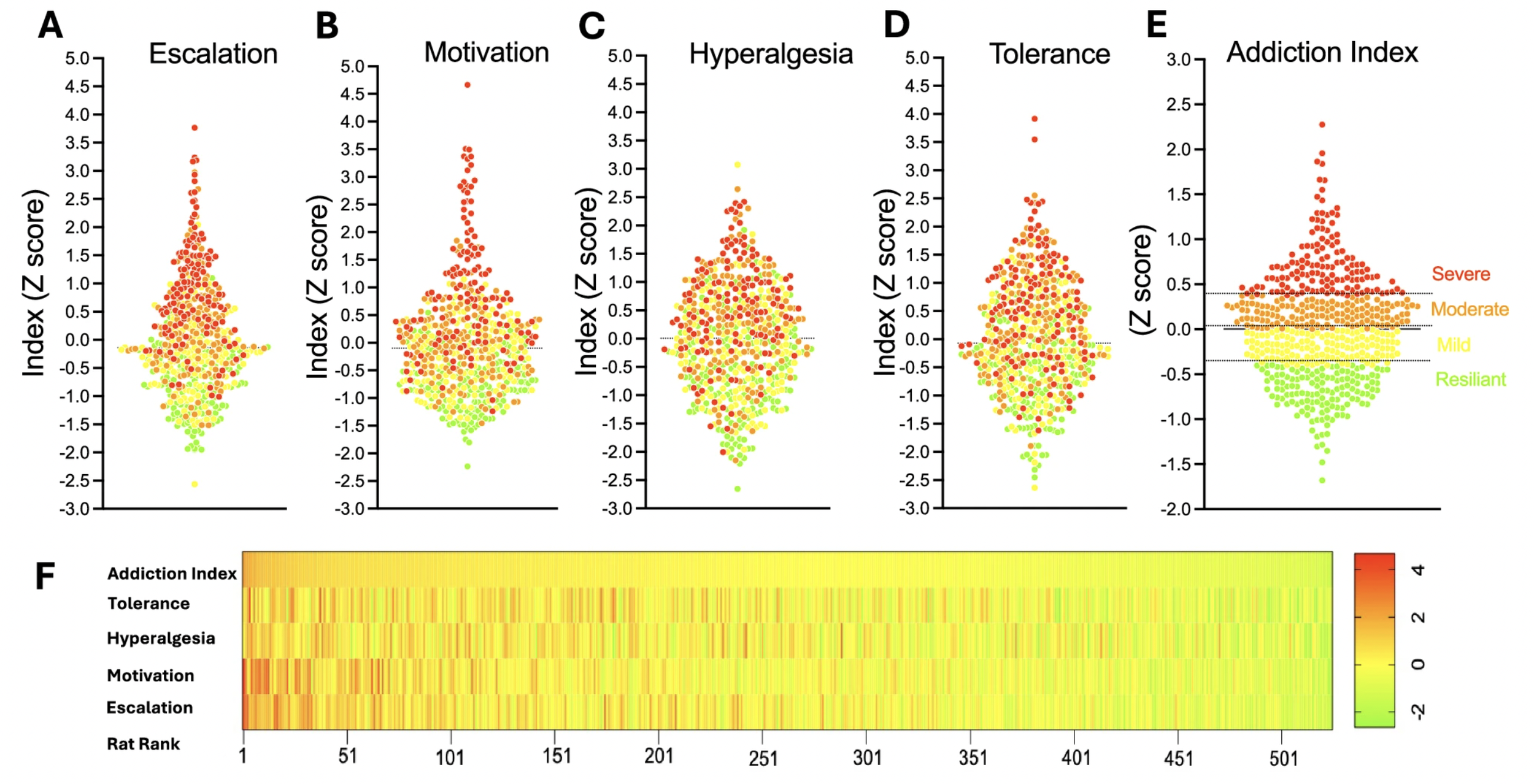
Large-scale Behavioral Characterization of Oxycodone Self-Administration in Heterogeneous Stock Rats Reveals Initial Analgesic Effects are Associated with Addiction-like Behaviors
In this study, Dr. Kallupi and the team performed a large-scale behavioral phenotyping of more than 500 genetically diverse heterogeneous stock rats to dissect individual vulnerability to oxycodone use disorder. Using extended-access intravenous self-administration combined with measures of escalation, motivation, analgesic tolerance, and withdrawal-induced hyperalgesia, developed a multidimensional Addiction Index that stratified animals into resilient and highly vulnerable phenotypes. We found marked inter-individual variability, with animals showing greater initial analgesic sensitivity exhibiting stronger escalation and addiction-like behaviors, and females displaying higher intake and motivation than males. This high-throughput, genetically informed framework establishes a powerful platform for genome-wide discovery of risk genes and translational targets for opioid addiction.
Perinatal Fentanyl Exposure drives Enduring Addiction Risk and Central Amygdala Gene Dysregulation
This work, led by Dr. Wood, describes a new rat model of perinatal fentanyl exposure in heterogeneous stock (HS) rats to examine Neonatal Opioid Withdrawal Syndrome (NOWS) and long-term addiction vulnerability. Offspring from fentanyl-exposed dams showed reduced survival, lower body weight, spontaneous withdrawal symptoms, and adolescent mechanical hypersensitivity, alongside negative affect. In adulthood, they exhibited increased fentanyl self-administration, heightened drug-seeking during reinstatement, and elevated corticosterone during withdrawal. RNA-seq of the central amygdala (CeA) revealed dysregulated pathways in G-protein coupled receptor signaling, adaptive immune response, and neurodevelopmental processes, providing mechanistic insights into enhanced addiction risk and stress reactivity. This model highlights translational relevance for mitigating NOWS and associated neuropsychiatric outcomes through targeted interventions. Neuropharmacology, 278 (2025) 110581.
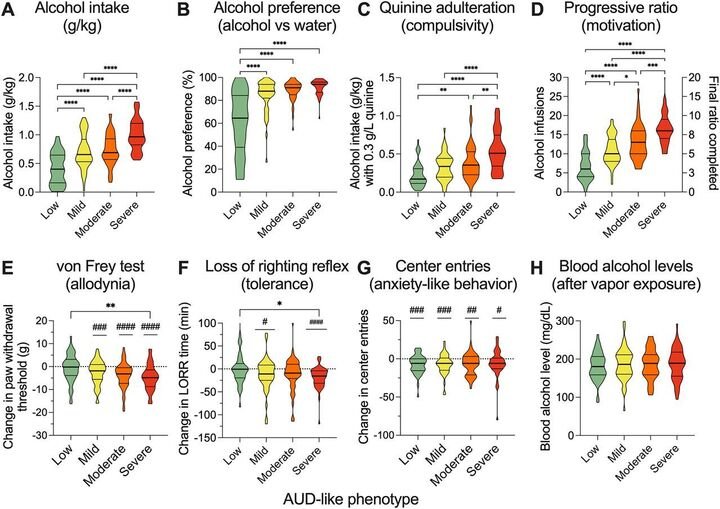
A Preclinical Alcohol Biobank: Samples from Behaviorally Characterized HS Rats for AUD Research
In this paper, led by Dr. Doyle, we describe the establishment of the Alcohol Biobank, a comprehensive repository of thousands of samples from over 700 genetically diverse heterogeneous stock (HS) rats, modeled after successful biobanks for cocaine and oxycodone. Using chronic intermittent ethanol vapor exposure (CIE) paired with oral self-administration, rats were characterized for AUD-like behaviors, including ethanol consumption, preference, motivation (progressive ratio), and withdrawal symptoms (e.g., allodynia, anxiety-like behavior). Longitudinal samples (blood, urine, feces) and terminal tissues (brain, heart, kidneys, liver, etc.) were collected at intoxication, acute withdrawal, protracted abstinence, or from naive controls, preserved via snap-freezing or fixation for multi-omics applications. This resource, freely available at www.alcoholbiobank.org to nonprofit organizations, facilitates biomarker discovery and therapeutic development by enabling access to well-characterized samples for researchers lacking behavioral infrastructure. eNeuro, September 2025, 12(9).
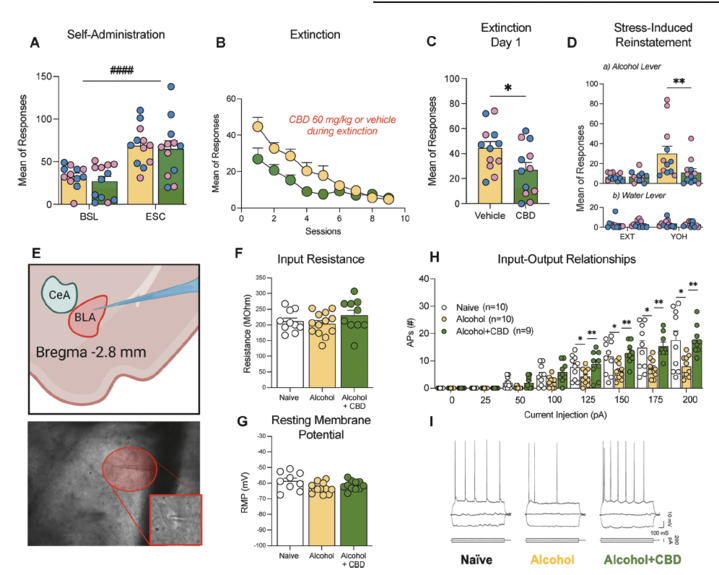
Cannabidiol Mitigates Alcohol Dependence and Withdrawal with Neuroprotective Effects in the Basolateral Amygdala and Striatum
Dirik, evaluated chronic cannabidiol (CBD) administration in two rodent models of alcohol dependence: chronic intermittent ethanol (CIE) exposure and ethanol vapor self-administration (EVSA). In the CIE model, CBD reduced alcohol self-administration during acute withdrawal, lowered motivation (progressive ratio), alleviated somatic withdrawal signs, anxiety-like behaviors, and mechanical hypersensitivity, while attenuating cue- and stress-induced reinstatement. Electrophysiological recordings showed CBD reversed alcohol-induced hyperexcitability in basolateral amygdala neurons. In the EVSA model, CBD decreased voluntary alcohol intake during escalation without affecting saccharin self-administration. Immunohistochemistry revealed CBD prevented alcohol-induced neurodegeneration in the nucleus accumbens shell and dorsomedial striatum. These findings underscore CBD's potential to modulate neuronal excitability and confer neuroprotection in AUD, informing mechanistic targets for pharmacotherapy. Neuropsychopharmacology, 2025 (published online).
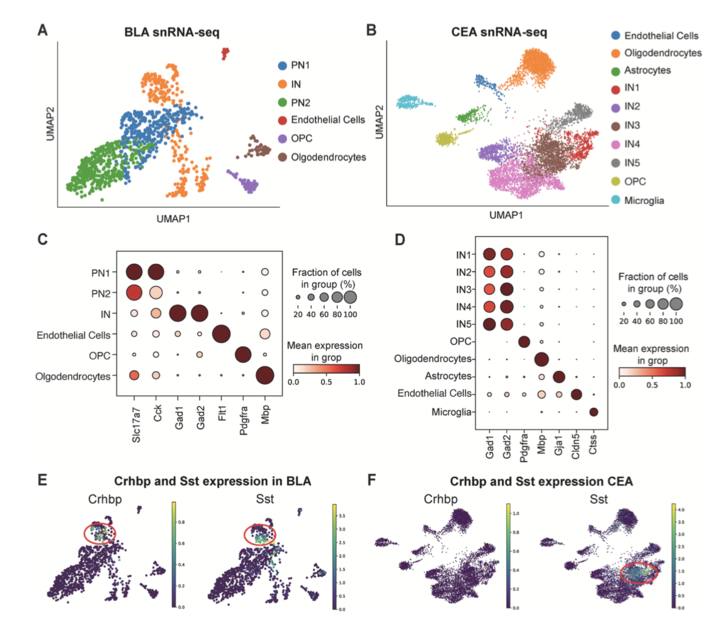
Corticotrophin-Releasing Hormone Binding Protein in the Basolateral Amygdala: Divergent Roles in Cocaine and Opioid Addiction like Behaviors
In this study, led by Dr. Wood, we investigated the role of corticotrophin-releasing hormone binding protein (CRHBP) in the basolateral amygdala (BLA) using viral-mediated knockdown, single-nucleus RNA sequencing (snRNA-seq), and operant self-administration paradigms in rats. snRNA-seq revealed Crhbp expression primarily in BLA somatostatin-positive interneurons. BLA Crhbp knockdown reduced cocaine self-administration and cue-induced reinstatement, attenuating stimulant-related behaviors, while conversely increasing oxycodone intake, progressive ratio breakpoints, and cue-induced reinstatement, enhancing opioid reinforcement and seeking. These substance-specific effects highlight CRHBP's divergent modulation of addiction neurocircuitry, positioning it as a candidate for tailored therapeutic interventions in cocaine and opioid use disorders. Addiction Neuroscience, 16 (2025) 100221.
Large-Scale Characterization of Cocaine Addiction-like Behaviors Reveals that Escalation of Intake, Aversion-Resistant Responding, and Breaking-Points are Highly Correlated Measures of the Same Construct
This study took an in-depth look at addiction-like behaviors in a large, genetically diverse sample of over 500 outbred rats. Using an extended cocaine self-administration paradigm, we analyzed individual differences across several core addiction measures: Escalation of drug intake over time, Motivation to obtain the drug (progressive ratio responding), continued drug use despite adverse consequences (foot shocks), and irritability-like withdrawal symptoms. By using this large sample size and extended drug access protocol, we were able to model addiction-like behaviors that more closely resemble the severity seen in human addiction. These findings provide important insights into the multidimensional nature of addiction and highlight the critical role that sex differences play in addiction vulnerability and resilience.
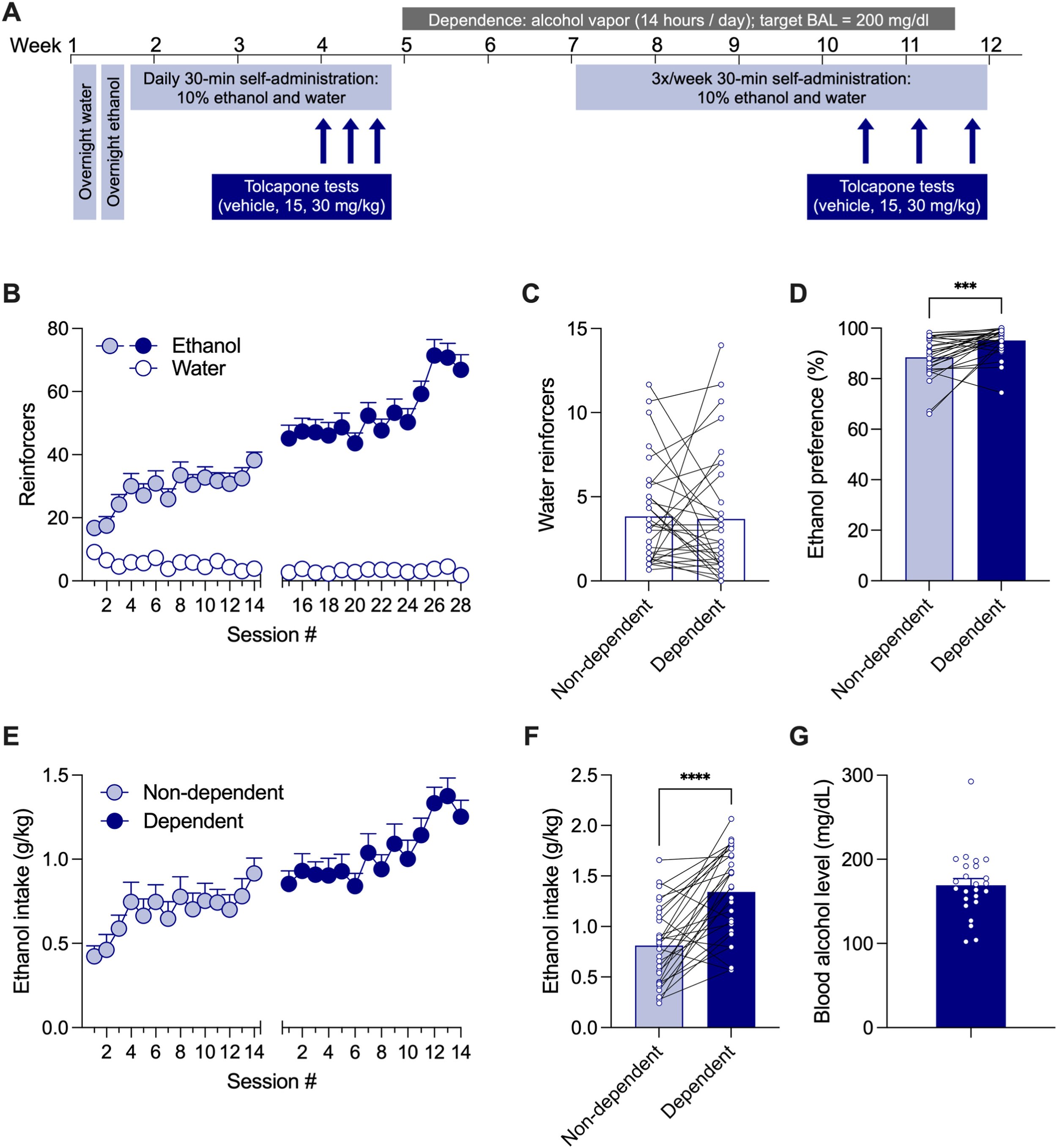
Catechol-O-Methyltransferase Inhibition and Alcohol Use Disorder (AUD): Evaluating the Efficacy of Tolcapone in Ethanol-Dependent Rats
One promising new approach to treat Alcohol Use Disorder (AUD) is the inhibition of the enzyme catechol-O-methyltransferase (COMT), which has shown potential to improve cognitive deficits commonly associated with AUD. The COMT inhibitor drug tolcapone, which is FDA-approved, has demonstrated some promise for treating AUD, but its ability to decrease drinking in alcohol-dependent animals has not been well-established. Here we found that Tolcapone attenuated ethanol self-administration in alcohol-dependent rats, but not in non-dependent animals or those self-administering saccharin. The effectiveness of tolcapone was influenced by the female rats' estrous cycle phase. These results suggest that COMT inhibition, specifically using tolcapone, may be a valuable pharmacotherapy for treating AUD, particularly in individuals who are physically dependent on alcohol.
Strain and Sex-Related Behavioral Variability of Oxycodone Dependence in Rats
Over the past two decades, the widespread prescription of opioid medications has led to a devastating opioid epidemic, with significant public health, social, and economic consequences. Improving treatments for opioid use disorder (OUD) requires a deeper understanding of its biological underpinnings, including the crucial role of genetic factors in individual susceptibility and clinical outcomes. In this study, we leveraged the genetic diversity of four different rat strains (ACI/N, BN/NHsd, WKY/N, and F344/N) to examine how genetic factors contribute to oxycodone metabolism and addiction-like behaviors. Using an extended access intravenous oxycodone self-administration procedure (12 hours/day), we characterized several key oxycodone-related measures: The findings revealed notable strain differences across several behavioral measures, as well as in oxycodone metabolism.
Nociceptin Attenuates the Escalation of Oxycodone Self-Administration by Normalizing CeA–GABA Transmission in Highly Addicted Rats
More than 2 million individuals in the United States currently have a substance use disorder that is related to prescription opioid pain relievers. We identified individual differences in oxycodone addiction-like behaviors in outbred heterogenous stock rats with high and low addiction-like behaviors. We found that the downregulation of nociceptin levels in the central nucleus of the amygdala (CeA) may be responsible for hyper–γ-aminobutyric acid (GABA)ergic tone in the CeA that is observed in individuals who develop addiction-like behaviors. The development of small molecules that target the nociceptin system may have therapeutic efficacy for the treatment of opioid use disorder.
Inactivation of a CRF-dependent Amygdalofugal Pathway Reverses Addiction-like Behaviors in Alcohol-Dependent Rats.
The activation of a neuronal ensemble in the central nucleus of the amygdala (CeA) during alcohol withdrawal has been hypothesized to induce high levels of alcohol drinking in dependent rats. In the present study we describe that the CeA neuronal ensemble that is activated by withdrawal from chronic alcohol exposure contains ~80% corticotropin-releasing factor (CRF) neurons and that the optogenetic inactivation of these CeA CRF+ neurons prevents recruitment of the neuronal ensemble, decreases the escalation of alcohol drinking, and decreases the intensity of somatic signs of withdrawal. Optogenetic dissection of the downstream neuronal pathways demonstrates that the reversal of addiction-like behaviors is observed after the inhibition of CeA CRF projections to the bed nucleus of the stria terminalis (BNST) and that inhibition of the CRFCeA-BNST pathway is mediated by inhibition of the CRF-CRF1 system and inhibition of BNST cell firing. These results suggest that the CRFCeA-BNST pathway could be targeted for the treatment of excessive drinking in alcohol use disorder.
Voluntary Induction and Maintenance of Alcohol Dependence in Rats Using Alcohol Vapor Self-Administration.
A major issue in the addiction field is the limited number of animal models of the voluntary induction and maintenance of alcohol dependence in outbred rats. To address this issue, we developed a novel apparatus that vaporizes alcohol for 2-10 min after an active nosepoke response. Male Wistar rats were allowed to self-administer alcohol vapor for 8 h/day every other day for 24 sessions (escalated) or eight sessions (non-escalated). Escalated and non-escalated rats were then tested for progressive ratio responding. Anxiety-like behavior, somatic signs of withdrawal, and hyperalgesia were assessed during acute withdrawal. The results showed that rats exhibited excellent discrimination between the active and inactive operanda (>85%), and the escalated rats quickly increased their blood alcohol levels from ~50 to >200 mg% in ~6 weeks. Compared with non-escalated rats, escalated rats exhibited severe addiction-like behavior, including somatic signs of withdrawal, anxiety-like behavior, hyperalgesia, and higher responding on a progressive ratio schedule of reinforcement. These results demonstrate that outbred rats will voluntarily self-administer alcohol vapor to the point of dependence without the use of forced alcohol administration, sweeteners, food/water restriction, operant pretraining, or behavioral/genetic selection. This novel animal model may be particularly useful for medication development to help unveil the neuronal circuitry that underlies the voluntary induction of alcohol addiction and identify novel molecular targets that are specifically recruited after the voluntary induction and maintenance of alcohol dependence.
Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence.
Alcohol dependence recruits neurons in the central nucleus of the amygdala (CeA). Here, we found that inactivation of a specific dependence-induced neuronal ensemble in the CeA reversed excessive alcohol drinking and somatic signs of alcohol dependence in rats. These results identify a critical neurobiological mechanism that is required for alcohol dependence, suggesting that targeting dependence neuronal ensembles may lead to a better understanding of the etiology of alcohol use disorders, with implications for diagnosis, prevention, and treatment.